(14.01.2020) Six leading European universities / research organizations, medtech companies, and software companies in 2017 formed a R&D consortium named MARIANA to create the most advanced image-guided flexible catheter platform on the market with immediate application to diagnosis and treatment of intraluminal disease such as lung cancer. Total budget was Euro 2.1m, split over 36 months.
The MARIANA project has now run for two years and is now moving into its third and final year (2020). A prototype of a brand-new system for lung cancer treatment that can address the challenge of reaching the outer lung regions is now ready for testing.
The complete system, code-named MARIANA NavSys consists of three subsystems:
- A new flexible catheter with high-precision integrated tracking and steering
- A new wireless tracking sensor
- A new user-friendly software platform for procedure planning and navigation
The sub-systems are fully integrated and form the basis of the MARIANA NavSys prototype. In the following we share some further details regarding the status of the prototype and technology behind the subsystems.
The catheter
The objective of the flexible catheter design has been to develop a one-hand operated instrument. Hence, during development of the catheter, focus has been on the user interface (handle) of the guidewire and guiding catheter, in addition to the steerability of the tip. Several different handles for controlling the guidewire and the catheter has been developed and tested. A final prototype design has passed through Design for Assembly and Design for Manufacturing procedures. Key characteristics of the new catheter is ease of operation and a low price compared to existing instruments on the market.
The tracking
The catheter and guidewire have been instrumented with high-precision tracking sensors for both spatial and rotational movement tracking. A new high-speed, high precision wireless tracking system has been developed and implemented with miniature sensors integrated into the catheter and in the guidewire tips. The tracking is controlled by a circuit board and the electromagnetic tracking field is set up by a field generator located on the table underneath the patient. The tracking is integrated with the navigation software and offers an extraordinary speed and precision during navigation.
The Software
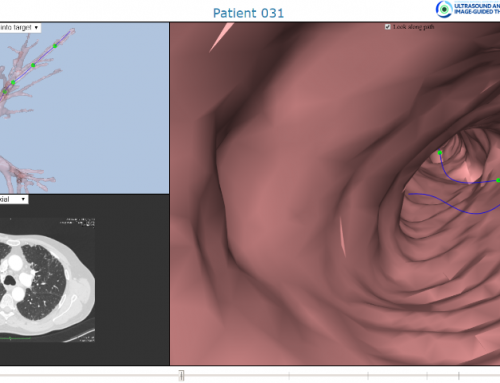
The navigation software is built on a web platform that allows for execution on entry level computer platforms. A novel web visualization engine gives the lung doctors the possibility to run the planning software from a web client such as Chrome, Safari or MS IE/Edge. This means that there is no longer need for expensive high-end workstations to run the MARIANA NavSys planning software, nor the navigation software in the operating room. All that is required in the operating room as an iPad with Bluetooth connection. A simplified User Interface and semi-automated data processing reduces the need for training on of the doctors to a minimum.
Figure 1: Screen dump of lung navigator, from development phase.
The Mariana consortium has started the search for investors to bring the Mariana system from prototype to commercial phase. A full validation and approval program has been launched and will run in parallel with the search for investors. The aim is to launch the commercial product in early 2023.
Figure 2: The system in use in operating room, pre-clinical trial.
Tor Helge Hansen
Managing Director.





Leave A Comment
You must be logged in to post a comment.